Author Archives: biochem4life
Last Biochem class *this semester*
So today was the last lap of the biochem class , it was quite informative , I enjoyed it. We went through the test paper (which i got an A in ^_^ ) , and saw the mistakes that were made , definitely going to fix those , i’m aiming for an A in biochem , and my incourse , lab and tutorial marks are all putting me in good stead. Now the real battle begins , *THE GRIND* to exams , this is where the fight is either won or lose , but im all about that W(win) , so im going to go as hard as possible.Till next time ……….good luck and study hard 😀 (GAMEFACE).
Just a random post
Waiting patiently for my Biochem Incourse results ………… Cant wait to see what i got 😀
A personal message
I know I have not been extremely personal in my blogs , but this might be the end of the course,so here goes . Let me first say that this blog was actually a lot fun to do , it never felt like work , rather it just felt like a place I could express how much I like biochemistry , also the fact that this blog is being marked is pretty cool , it brings mark allocation and student evaluation out of the exam room , it gives us a chance to think , express ourselves , postulate ideas and overall indulge in the awesomeness that is Biochemistry. I think that this blog , shows that extra-exam room (outside of the exam room) evaluation works , it really does , I feel that this is the future of learning , online videos , blogs , pod casts , these are all fun ways of learning , I personally think that what this course is doing , is the future of learning and I am extremely glad that I can be part of it and experience it first hand , so whoever may be reading this right now , If u are a student then I hope you have enjoyed this as much as I have , if you are a visitor let me just say that biochemistry is an awesome subject and you should definitely give it a try (http://www.youtube.com/user/BiochemJM is a nice place to start) , and finally if you are an alien , then come say hello at the St Augustine Campus of UWI in Trinidad.
Best Wishes,
Andre R.
Lipids
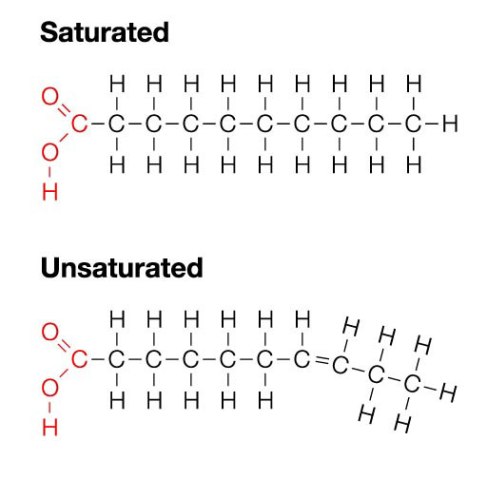
There are many different classes of lipids , such as fatty acids , triacyglycerols , phospholipids , steroids and glycolipids. Fats are solid at room temperature and oils are liquids at room temperature , this is because fats contain saturated hydrocarbon chains whereas oils contain unsaturated hydrocarbon chains , meaning that there is at least one carbon to carbon double bond In the chain. Fats are very important and have many different functions and properties , they are a source of energy , giving nine kilocalories per gram , they are an energy reserve meaning that any extra energy derived from food is stored as fat , they can provide insulation and protection to internal organs.
Saturated fats are fats which contain no double bonds , therefore all the carbons atoms are bonded to bonded to hydrogen atoms. Saturated fats contain long straight chains , they are solid at room temperature and are mostly animal fats.
Unsaturated Fats have carbon to carbon double bonds . they are found in plants and fish , and vegetable based oils , they are liquid at room temperatures and their structures contain kinks caused by the double bonds.
Naturally occurring fatty acids are found to have even numbers of carbons, and the double bonds are in the cis form. It must also be noted that the presence and number of carbon doubles decreases the melting point. This is why if u take a tub of butter that claims to contain no saturated fats , meaning it only contains unsaturated fats , and leave it at room temperature you would find it turns into an oily , greasy pool inside in the tub, hence this why manufacturers of butters and margarines made of unsaturated fats put a disclaimer on the tub saying , keep refrigerated.
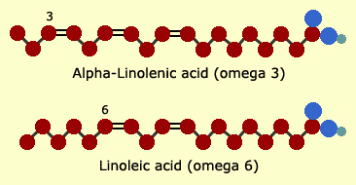
Non essential fatty acids are fatty acids which our body can synthesize and are therefore not required to come from our diet , alternatively essential fatty acids are fatty acids which cannot be synthesized in the body and must come from our diet. Some examples of essential fatty acids are omega-6 linoleic acid and omega-3 alpha-lenoleic acid.
The iodine index is used as the degree of unsaturation and can be found by measuring the amount of iodine that reacts with the unsaturated fat or oil.
References:
Pictures taken from
1) The BIOCHEMJM Youtube channel
http://www.youtube.com/watch?v=kDTp_cS5po4&feature=youtu.be
2) http://www.arcadia.co.za/shop/omega-3-plus-capsules/
3) http://www.webmd.boots.com/diet/best-worst-foods-belly-fat-rm-quiz
The TCA cycle
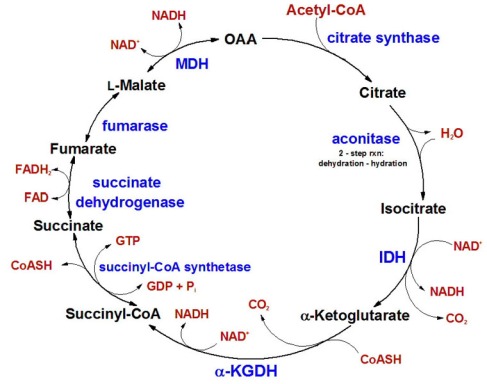
The TCA cycle or Tricarboxylic acid cycle , also known as the Krebs cycle is a compilation of chemical reactions occurring under aerobic conditions to generate energy. The cycle is made up of 8 steps and is catalysed by a number of enzymes. The TCA cycle occurs in the matrix of the mitochondria of eukaryotic cells and in the cytosol of Bacteria which lack mitochondria. The link between glucose and the citric acid cycle is this , when glucose enters glycolysis it is converted to two molecules of pyruvate , these pyruvate molecules are then converted to Acetyl CoA via the link reaction which enters the Kreb’s and cycle continues . The TCA or Kreb’s cycle can be seen below;
Picture taken from
The Medical Biochemistry Page Online
Glycolysis
We now enter the wonderful topic of Glcolysis , which is my favourite topic , glycolysis consists of 10 enzyme catalysed reactions. The first five reactions of glycolysis are what is referred to as the energy investment phase , since ATP is invested into the reactions , the last five reactions are known as the energy payoff phase since it is here that the ATP used is regenerated with two extra ATP molecules , giving glycolysis a net gain of two ATP per molecule of glucose used. It must be said however that the real stars of glycolysis are the enzyme since they allow the reactions to be fast and energy efficient.
1st Reaction
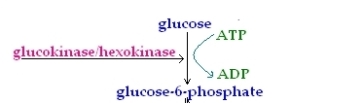
This is the first Priming reaction of glycolysis and the first reaction of the energy investment phase as well. The reaction is irreversible. In this reaction Glucose is converted to Glucose-6-phosphate. The reaction uses an ATP molecule and converts it to ADP , the enzyme which catalyses the reaction is Hexokinase.
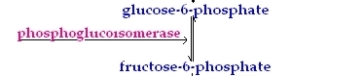
2nd Reaction
This reaction converts Glucose -6-phophate to Fructose-6-phosphate via the enzyme Phosphohexose isomerise.
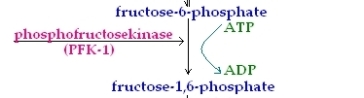
3rd Reaction
This reaction is the second of two irreversible reactions which occur in the energy investment phase. This reaction is the second priming reaction and consumes an ATP molecule whoch is converted to ADP , in this reaction Fructose-6-phosphate is converted to Fructose-1.6-Bisphosphate via the enzyme Phospho-fructokinase-1 (PFK-1). It must also be noted that this is the most regulated step of glycolysis.
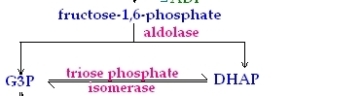
4th Reaction
This is the splitting reaction , in this reaction the six carbon molecule Fructose-1.6-bisphosphate is converted to two three carbon molecules , one is called Glyceraldehyde -3-phosphate and the other is called Dihydroxyacetone phosphate. The enzyme which catalyses this reaction is Aldolase.
5th Reaction
This is the final reaction of the energy investment phase , in this reaction the Dihydroxyacetone phosphate is converted to another molecule of glyceraldehyde-3-phosphate via the enzyme Triose phosphate isomerise. Hence when the energy payoff phase begins , there will be two molecules to glyceraldehydes-3-phosphates to be used.
ENERGY PAYOFF PHASE
6th reaction
In this , the first of the energy payoff reactions , an inorganic phosphate is used , as well as an NAD+ molecule which is converted to NADH and a proton. In this reaction the two molecules of glyceraldehyde-3-phosphate are converted to two molecules of 1,3-Bisphosphoglycerate via the enzyme Glyceraldehyde-3-phophate dehydrogenase.
7th Reaction
In this reaction two ATP molecules are generated from two ADP molecules , the reaction converts two molecules of 1,3-bisphosphoglycerate to two molecules of 3-phosphoglycerate , via the enzyme Phophsglycerate kinase.
8th Reaction
In this reaction the two molecules of 3-phosphoglycerate are converted to two molecules of 2-phophogycerate via the enzyme Phophoglycerate mutase.
9th Reaction
In this reaction two molecules of water are removed , the two molecules of 2-phosphoglycerate are converted to two molecules of Phosphophenolpyruvate via the enzyme Enolase.
10th Reaction
This is the final reaction in glycolysis , it generates two molecules of ATP from two molecules of ADP. In this reaction two molecules of Phosphophenolpyruvate are converted to two molecules of pyruvate via the enzyme Pyruvate kinase. It should be noted that this is the only irreversible reaction in the energy payoff phase of glycolysis.
It can be seen therefore that since two ATP molecules are used in the energy investment phase and Four ATP molecules are produced in the energy payoff phase , that there is a net gain of two ATP molecules from one glucose molecules from glycolysis.
So what happens to pyruvate after it’s produced? What’s the Fate of pyruvate?
Pyruvate has three main fates;
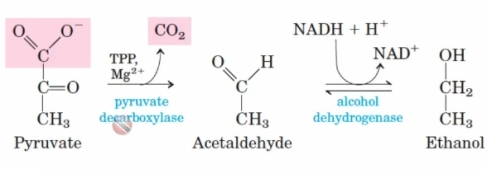
1) pyruvate under anaerobic conditions to produce ethanol and carbon dioxide , whereby , , pyruvate is converted to acetaldehyde via the enzyme pyruvate decarboxylase using cofactors TPP and Mg2+ ions , this reaction produces carbon dioxide as a by product . The acetaldehyde is then converted to ethanol via the enzyme alcohol dehydrogenase , this reaction also converts NADH to NAD+.
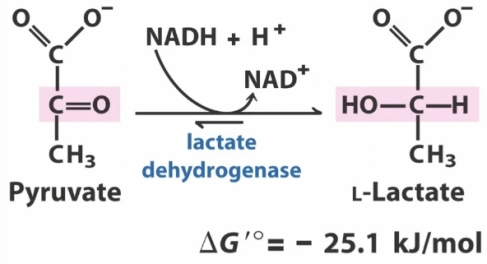
2) pyruvate under anaerobic conditions to produce lactate ,this reaction occurs via the enzyme lactate dehydrogenase and in this reaction NADH is converted to NAD+. Lactate is produced in vigorously contracting muscles because in such circumstances there is an oxygen debt present and hence anaerobic conditions would be prevalent , and in erythrocytes because they lack mitochondria to produce energy otherwise.
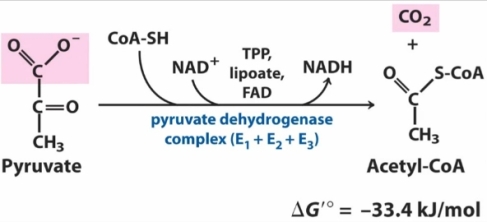
3) pyruvate under aerobic conditions to produce carbon dioxide and water. The first stage of this reaction is converting pyruvate to Acetyl-CoA , this reaction occurs via an enzyme complex referred to as the pyruvate dehydrogenase complex , which consists of three enzymes , this reaction produces carbon dioxide and uses CoA-SH as a cofactor , in the reaction NAD+ is converted to NADH. The second stage of the conversion occurs via the citric acid cycle where the Acetyl-CoA is converted to carbon dioxide and water.
It must also be noted that Fermentation ie. Fates 1 and 2 are used to regenerate NAD+ to be used in glycolysis.
References:
Pictures taken from
1) The BIOCHEMJM Youtube channel
http://www.youtube.com/watch?v=K-NMuq-XIHo
2) Windsor Education Online
http://windsor.edu/Assi_model.htm
Enzymes
So we have come to what in my mind is the most important topic of the course , yes , enzymes , these little protein buddies , as I like to call them , speed up chemical reactions within our body and allow us to live.
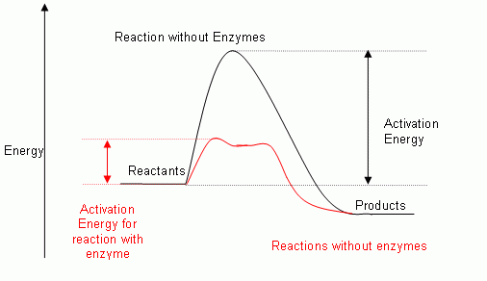
Enzymes are biological catalysts , hence they provide an alternative pathway to a reaction with a lower activation energy , activation is the minimum amount of energy required to initiate a chemical reaction. It should be known that enzymes do no change the free energies of reactants or products and does not change the equilibrium either. An energy profile diagram for an enzyme catalyzed reaction can be seen below.
The rate at which enzymes work can be expressed via the turnover number or Kcat , this is the number of molecules of substrate converted into product per enzyme per second. Naming enzymes allows us to identify the classes of enzymes by a single number , there are six classes of enzymes , 1)Oxidoreductases , 2) Transferases, 3) Hydrolases, 4) Lyases ,5) Isomerases, 6) Ligases.
Cofactors , a cofactor is a non protein component that makes the enzyme work . Prosthetic groups are coenzymes which are permanently associated , while Co substrates are transiently associated.
Apoenzyme + Cofator —– > Holoenzyme
Apoenzyme – the inactive protein part
Cofactor- non protein part
Holoenzyme – active protein
Active sites are the sites which the substrates bind to on the enzyme , the active site is usually a pocket of cleft. Interactions between the active site and subsrate occur via the same forces which stabilize the protein structure , ie. Hydrogen bonding , Van Der Waals interactions , hydrophobic interactions and electrostatic interactions.
There are a couple of hypothesis regarding the substrate , enzyme interaction. One such hypothesis is the Lock and Key hypothesis , which states that the active site of an enzyme is completely complementary in shape to the substrate it is catalysing. The Kushland’s Induced Fit hypothesis states that the shape of an enzyme active site is not exactly complementary to the shape if the substrate but rather it changes slightly in shape to accomadate the substrate , hence inducing its fit.
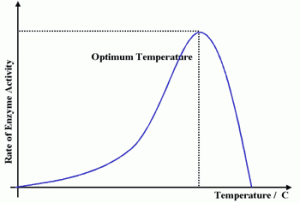
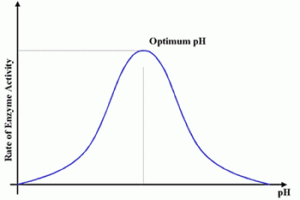
The following are graphs showing how different conditions , heat , temp etc , affect the rate of an enzyme catalyzed reaction.
As we have seen from the graphs above , enzymes are temperature sensitive and will denature after a certain has been reached. Enzymes have a higher pH tolerance because of their internal hydrophobic interactions , hence their needs to be a substantial change in pH to destabilize the structure and denature the enzyme.
This brings us now to inhibitors , an inhibitor is any substance which diminishes the velocity of an enzyme catalysed reaction. There are different types of inhibitors that affect enzymes and they all have different ways in which they affect the rate of reaction.
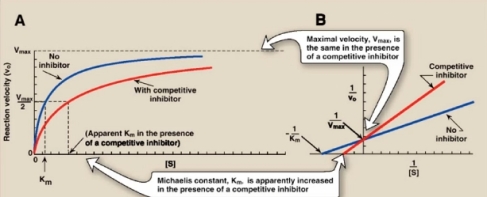
Competitive inhibition – the inhibitor binds to the same site as the substrate would normally occupy. The line-weaver Burk plot and Michaelis Menten curve for this type of inhibition is seen below.
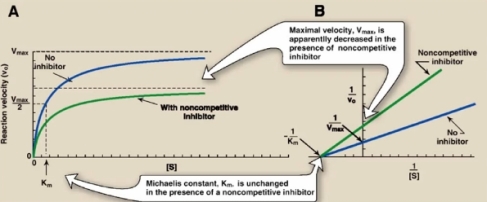
Non competitive inhibition- The inhibitor binds to different sites on the enzyme , and the inhibitor can bind to either a free enzyme of the enzyme-substrate complex. The Line-Weaver Burk Plot and Michaelis Menten curve for this type of inhibition is seen below.
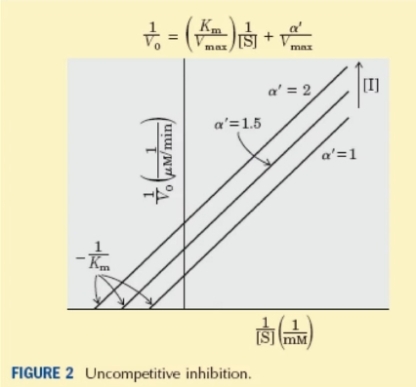
Uncompetitive inhibition – The inhibitor binds to the enzyme-substrate complex , at a separate site from the substrate active site and not with the free enzyme.The The Line-Weaver Burk Plot for this type of inhibition is seen below.
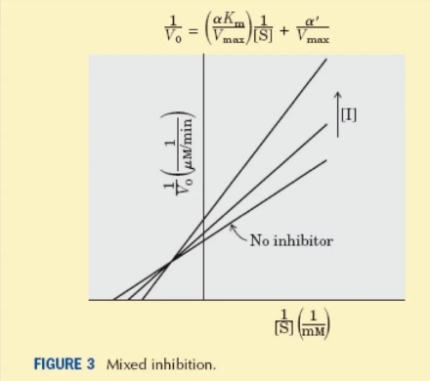
Mixed inhibition- This occurs when the inhibitor binds at a separate site from the substrate active site , to either the free enzyme or the enzyme-substrate complex. The Line-Weaver Burk Plot for this type of inhibition is seen below.
We come now to the last point of this topic , which is Allosteric enzymes , they have more than one active site which cooperatively binds substrate molecules. The binding of one molecule causes a conformational change in the enzyme causing the other active site to gain an affinity to substrate and catalyse them.
References:
Pictures taken from ;
The BIOCHEMJM Youtube channel.
Revision World- Biology
http://revisionworld.co.uk/gcse-revision/biology/cell-activity/proteins-and-amino-acids/enzymes
Video Review 2 – Cells
http://www.youtube.com/watch?v=cj8dDTHGJBY
This video was taken from the crash course Youtube channel and it is about cells , animal cells to be precise. The video contains 10 main points as seen in the recap at 11:23 in the video. The first point in the video deals with Robert Hooke , the scientist who did the first research on cells , this is a good strategy , it employs history as a tool in the learning process , and gives a background to the topic at hand. The second point deals with cilia and flagella and discusses how in certain cells , they are used for movement. The video gives examples of human cells which have cilia , ie lung cells and human cells which have flagella ie sperm cells , this gives the video a more relatable tone. The third point discusses the cell membrane and it is at this point that the video starts describing the cells as a type of city , run by a dictatorship , this helps the viewer develop a better idea of the topic. Describing cells as a city or country seems to be a very popular tool in teaching this topic as I have seen many videos employing this very technique. Points five to nine goes into the various organelles that can be found within a cell , endoplasmic reticulum both smooth and rough which take part in steroid synthesis and protein synthesis , ribosomes which can attach to mRNA, golgi apparatus which transports proteins and enzymes , lysosomes molecules which break down molecules within the cell and the nucleus which contains the genetic layout of the cell. In the last point the host describes what he states as his favourite organelle , the mitochondria , the video discusses briefly the role of mitochondria which is to provide energy to the cell . During the last point the video also discusses the endosymbiotic theory , which states that organelles such as mitochondria were once free living organisms and were engulfed by other organisms , the video also goes into the evidence of this, being that mitochondria have their own DNA , and also discusses how this information can be used to trace back family trees since the mitochondrial DNA within a child is identical the mitochondrial DNA in the mother.
Overall I enjoyed this video , however I do have some critiques about it , one such critique is that the video is not nearly as informative as a proper science video should be , I found it lacking in information. Another problem I had with the video was that it seemed to have very subjective tone used , meaning that a lot of opinions were used , another problem is that the video makes a lot of references to past videos , giving links every which way , this is confusing for a first time viewer of the channel and also means that someone will not be able to watch just that one video and have a decent understanding of cells. There were also things that I did quite like , for one the style of the video is very visual , there are a lot of pictures and video clips of animals and even animations showing how certain parts of the cells work , this keeps the attention of the viewer and would make them more likely to watch the entire thing . Also the fact that the video is short , encourages people to watch the video.
In conclusion , although the video was very entertaining with its animations and clips , it must be said that it lacked heavily in substance and does not fully explain how the cells works or even the proper functions of the organelles, therefore I would not recommend this video to a biochemistry student as they would find it non informative. I think the target audience for this video would be to a form 1 class of students who are just starting to learn about science , as it gives a simplistic and elementary view of the cell.
Video Review 1 – Glycolysis Part 2: Fates of Pyruvate
Video Review 1 – Glycolysis Part 2: Fates of Pyruvate
http://www.youtube.com/watch?v=K-NMuq-XIHo
This video was taken from the BIOCHEMJM Youtube channel. First of all , I must say that this topic is my favourite topic throughout this course , this video goes into detail about what happens after the pyruvate is produced during glycolysis. Oddly enough looking through youtube , although a lot of videos regarding Glycolysis were found , videos detailing the use of pyruvate , or the fates of pyruvate were few and far in between , hence from my point of view I would regard this video as the best and most in depth one that I’ve seen concerning the topic so far. The video has three main points , 1) the use of pyruvate under anaerobic conditions to produce ethanol and carbon dioxide , 2) the use of pyruvate under anaerobic conditions to produce lactate and 3) the use of pyruvate under aerobic conditions to produce carbon dioxide and water. In the first point the video makes it goes into fermentation and lists the importance of fermentation , stating that it replenishes NAD+ for use in glycolysis. The videos goes into the reaction whereby , under anaerobic conditions , pyruvate is converted to acetaldehyde via the enzyme pyruvate decarboxylase and the use of the cofactors TPP and Mg2+ ions , this reaction produces carbon dioxide as a by product . The acetaldehyde is then converted to ethanol via the enzyme alcohol dehydrogenase , this reaction also converts NADH to NAD+. The second point made is the conversion of pyruvate to lactate under anaerobic conditions , the reaction occurs via the enzyme lactate dehydrogenase and in this reaction NADH is converted to NAD+. Lactate is produced in vigorously contracting muscles because in such circumstances there is an oxygen debt present and hence anaerobic conditions would be prevalent , and in erythrocytes because they lack mitochondria to produce energy otherwise. The third point discussed is the conversion of pyruvate to water and carbon dioxide under aerobic conditions. The first stage of this reaction is converting pyruvate to Acetyl-CoA , this reaction occurs via an enzyme complex referred to as the pyruvate dehydrogenase complex , which consists of three enzymes , this reaction produces carbon dioxide and uses CoA-SH as a cofactor , it was also noted in the video that in the reaction NAD+ is converted to NADH. The second stage of the conversion occurs via the citric acid cycle where the Acetyl-CoA is converted to carbon dioxide and water. Overall I must say that I enjoyed the video for a number of reasons ,such as , the length , the video is roughly about eighteen minutes long , this is GOOD because it allows a maximum of information to be absorbed in a smaller period of time , it also means that if one were to want to re watch the video , it wouldn’t seem like such a task , also what I have found is that because the download size of the video is relatively small and would not take up much memory , people are more inclined to put the video on their phone and watch in their free time to refresh themselves on the topic , this is what I personally do. Another reason I like this video is because after watching this video and attempting an online quiz I scored 94.5% , which is also GOOD , I think that the fates of pyruvate is an important topic since it explains why glycolysis even occurs to produce pyruvate in the first place. If I had to make a recommendation to better the video , I would include more dynamic slides or video clips , since the lecture is in a video format it allows moving images and flashy effects to be used , I think such inclusions would better help to keep the attention of the viewer.
In conclusion , the video is extremely informative , well produced , formatted nicely , it is interesting , flows one point into the other and is overall a great resource for anyone interested in glycolysis or biochemistry.
Multiple Choice Questions ^______^
i) In amino acids , a zwitterion is formed when;
A) The carboxylic group donates a proton to the R group.
B) A hydrogen from the amino group is lost as proton and accepted by the carboxylic group.
C) The R group donates a proton to the carboxylic group.
D) The amino group donates a proton to the R group
E) A hydrogen from the carboxylic group is lost as a proton and accepted by the amino group.
ii) Which of the Following amino acids have aromatic R groups?
1) Serine
2) Phenylalanine
3) Lysine
4) Tyrosine
A) 1,2 and 3 only
B) 1 and 3 only
C) 2 and 4 only
D) Only 4
E) All are correct
iii) Which of the following enzymes is responsible for the splitting in glycolysis?
A) Aldolase
B) Enolase
C) Pyruvate Kinase
D) PFK-1
E) Hexokinase
iv) Which of the following enzymes is the most regulated in glycolysis
A) Hexokinase
B) Aldolase
C) Phospho-fructokinase-1
D) Enolase
E) Pyruvate kinase
v) Which of the following organelles contain DNA
1) Mitochondria
2) Chloroplasts
3) Vacuole
4) Nucleus
A) 1,2,3 only
B) 1 and 2 only
C) 1,2 and 4 only
D) 4 only
E) All are correct
vi) Which of the following are disaccharides?
1) Lactose
2) Sucrose
3) Maltose
4) Fructose
A) 1,2 and 3
B) 1 and 2 only
C) 1,2 and 4 only
D) 4 only
E) All are correct
vii) A zwitterions has a net charge of;
A) 1+
B) 2+
C) 0
D) 1-
E) 2-
viii) The biuret test is used to identify;
A) Proteins
B) Amino acids
C) Carbohydrates
D) Reducing sugars
E) None of the above
ix) The Ninhydrin test is used to identify;
A) Proteins
B) Amino acids
C) Carbohydrates
D) Reducing sugars
E) None of the above
x) What is the net gain during glycolysis?
A) 2 ATP
B) 4 ATP
C) 2 ATP and NADH
D) 4 ATP and 4 NAD+
E) 4 NADH
xi) Which of the following is not found in animal cells?
A) Nucleus
B) Mitochondria
C) Chloroplast
D) Rough ER
E) Cell Membrane
xii) How many irreversible reactions are in the energy investment phase of glycolysis?
A) 2
B) 1
C) 3
D) 0
E) None of the above
xiii) Which enzyme is used in the 1st priming reaction of glycolysis?
A) PFK-1
B) Hexokinase
C) Aldolase
D) Enolase
E) Pyruvate kinase
xiv) How many ATP molecules are used in the energy investment phase of glycolysis?
A) 1
B) 2
C) 3
D) 4
E) None of the above
xv) Sucrose is a non-reducing sugar?
[] True
[] False
xvi) The R group in Glycine is H , a single hydrogen atom.
[] True
[] False
xvii) Uncompetitive enzymes bind to the substrate active site.
[]True
[] False
xviii) The primary structure of a protein refers to the;
A) Folding of the structure
B) Type of bonds holding the protein together
C) The sequence of amino acids making up the protein
D) Structure being in an alpha helix or beta pleated sheet
E) None of the above
xix) Which of the following is true about Globular proteins?
1) They are water soluble.
2) They function in dynamic roles.
3) They can function as enzymes.
4) They have one dominating secondary structure.
A) 1,2 and 3 only
B) 1 and 2 only
C) 2 and 4 only
D) 1 and 3 only
E) All of the above
xx) Which of the following amino acids is not allowed in an alpha helix?
A) Serine
B) Alanine
C) Glutamine
D) Glycine
E) Valine